Breaking Through the Latest Breakthroughs.
Immuno-oncology has taken center stage in innovation in cancer treatment in recent years. Immunotherapy is the latest breakthrough, becoming an effective tool in prolonging the lives of patients with many different kinds of cancer. But while immunotherapy is effective in some cancers, it is not a universal tool, nor is it without its drawbacks, including severe and unpredictable toxicities. More research must be done—and innovative points of view must be explored—to unlock the next breakthrough. This is the heart of our work at the Duncan and Nancy MacMillan Cancer Immunology and Metabolism Center of Excellence at Rutgers Cancer Institute.
Manipulating Tumor Metabolism to Target New Cancer Therapies.
Metabolism controls the function of the immune system in many ways that can be modulated to improve anti-cancer immune responses. The metabolism of the immune cells themselves controls their tumor-killing ability. Metabolic changes are an essential aspect of tumorigenesis (the process by which normal cells become cancer cells), and these very changes produce materials in the tumor microenvironment (the cells, molecules, and structures that make up the tumor) that can suppress anti-cancer immune responses. This interplay between immune activation and metabolites in the tumor microenvironment provides the framework for an important new avenue of cancer research, one being led by Dr. Eileen White, as one of the bases and primary research endeavors for the Duncan and Nancy MacMillan Cancer Immunology and Metabolism Center of Excellence.
Making Remarkable Discoveries, Now and Tomorrow.
Researchers at the Duncan and Nancy MacMillan Cancer Immunology and Metabolism Center of Excellence made the discovery that, in order to meet their demand for nutrients to support growth, tumors engage in nutrient “scavenging,” by which tumors grab molecular nutrients (macromolecules) from inside and outside the tumor cell. These macromolecules are used as metabolic fuel for tumor growth and survival. Thus, nutrient availability and tumor metabolism play a major role in tumor growth that can be targeted for cancer therapy.
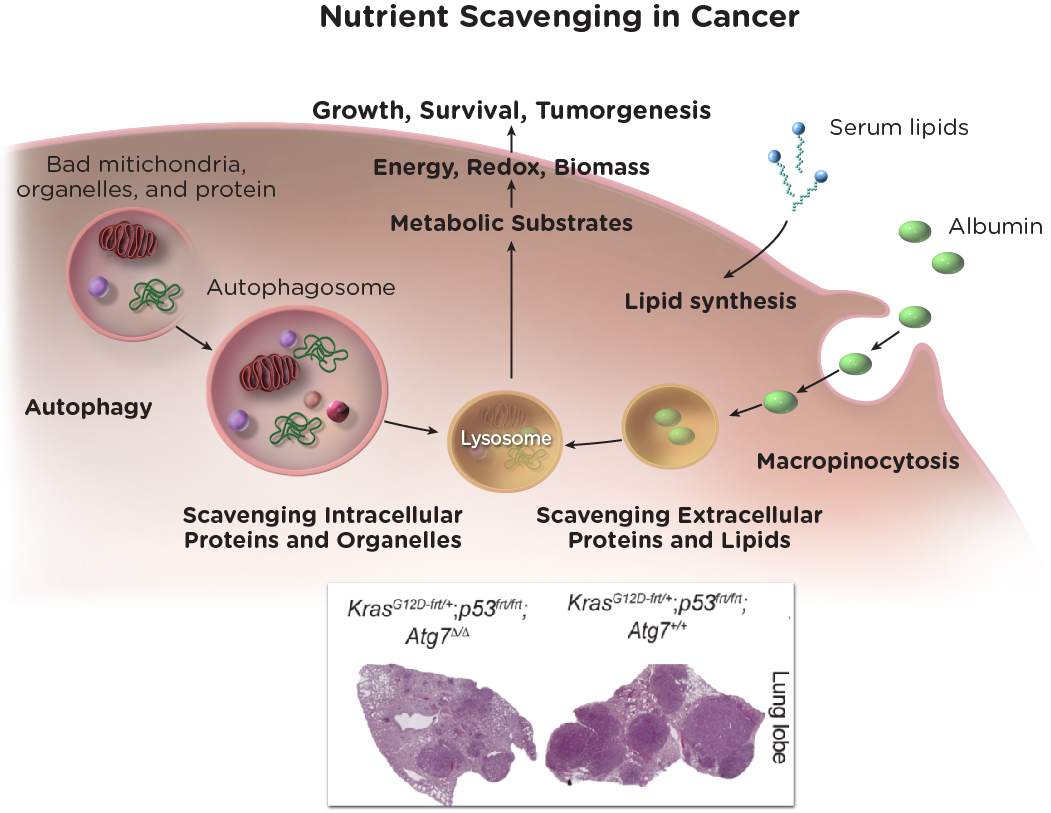
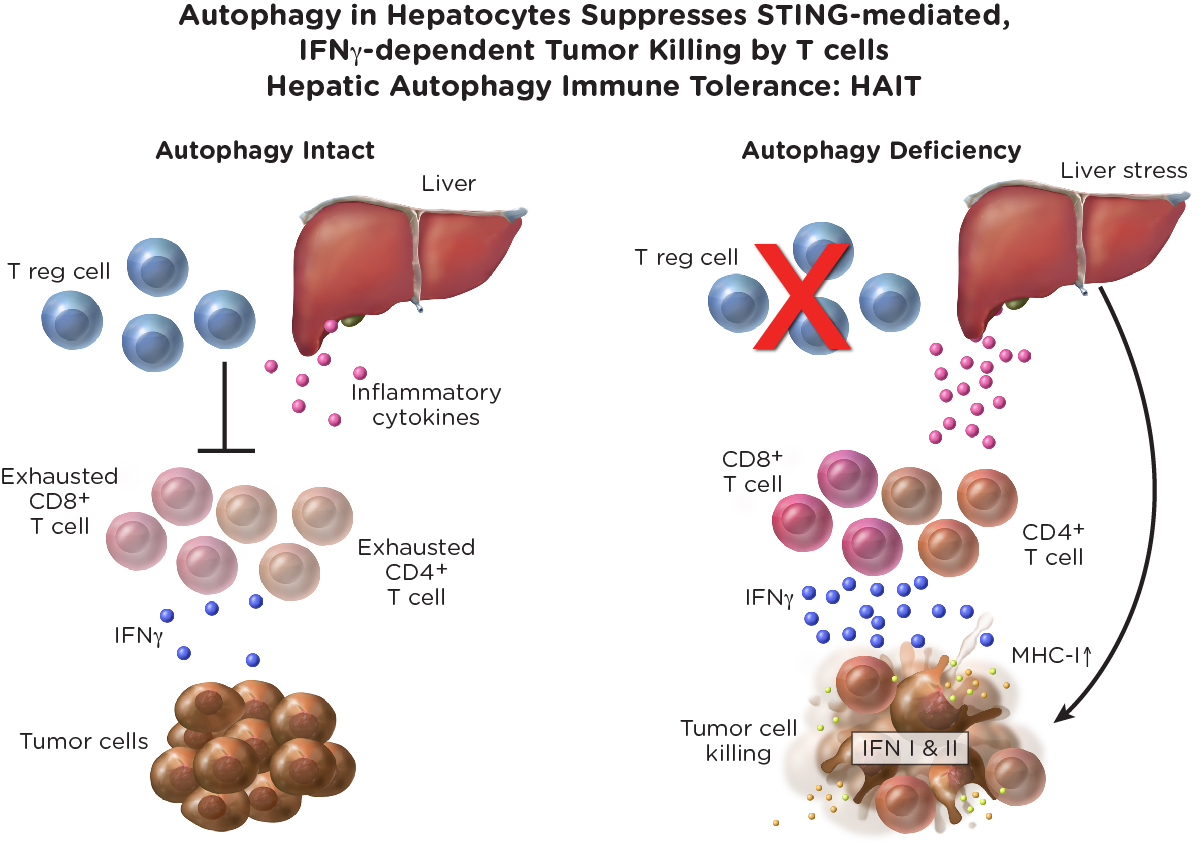
Rutgers Cancer Institute researchers established that nutrient scavenging by autophagy—a process by which cells degrade and recycle their intercellular components to sustain metabolism and survival—suppresses interferon pathway activation and inflammation and in turn, limits an anticancer T cell response. These findings demonstrate that by suppressing an innate immune response and interferon production, autophagy suppresses tumor killing by T cells. Thus, inhibiting autophagy is a new means to promote an anti-tumor immune response and also may increase response to immunotherapies.
The following diagram presents the concept of how the loss of nutrient scavenging by autophagy promotes an anticancer T-cell response, which can help suppress the growth of cancer cells.
The research of the Duncan and Nancy MacMillan Cancer Immunology and Metabolism Center of Excellence is poised to secure the link between immunology and cancer metabolism, leading to the next breakthrough in cancer therapy.
Find Out More.
To learn more about our programs and research, please see the following:
- For more information about our clinical trials call 732-235-7356 or search for a clinical trial.
- For inquiries about employment opportunities, please click here.
- If you are interested in supporting the Duncan and Nancy MacMillan Cancer Immunology and Metabolism Center of Excellence at Rutgers Cancer Institute, please contact the Rutgers Cancer Institute Development Office at cinjdevelopment@ruf.rutgers.edu.

