Research Overview
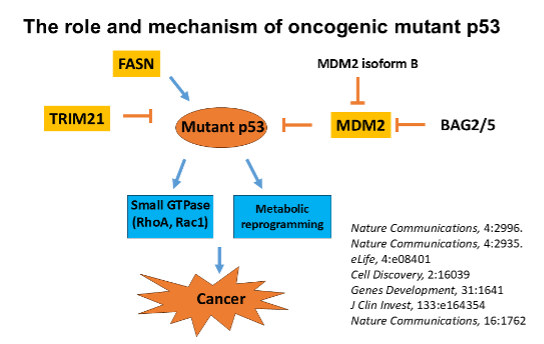
p53 is a key Tumor suppressor. p53 is the most frequently mutated gene in human tumors; over 50% of all human tumors harbor mutations in the p53 gene. As a transcription factor, p53 transcribes its target genes to regulate various cellular responses to exert its function in tumor suppression. Many tumor-associated mutant p53 proteins not only lose the tumor-suppressive function but also acquire new activities that promote tumorigenesis, known as oncogenic mutant p53. We have a long-standing interest in studying the p53 signaling pathway and oncogenic mutant p53 in cancer.
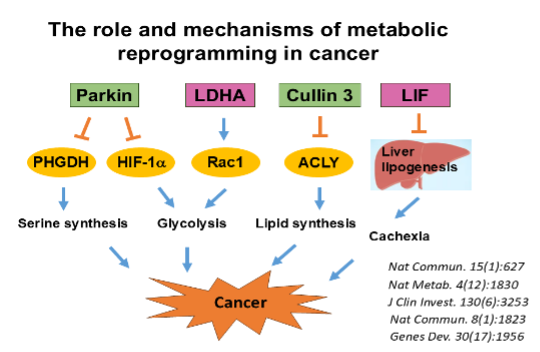
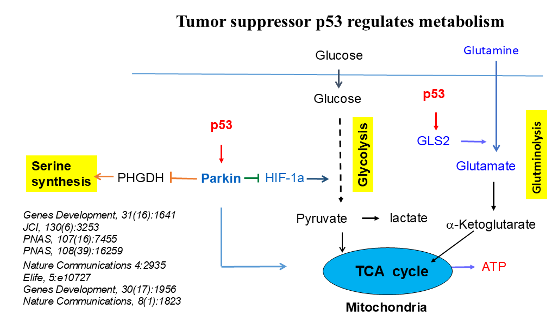
Metabolic reprogramming is a hallmark of cancer cells and a key contributor to cancer progression, which is a promising target for cancer therapy. We are interested in studying mechanisms underlying metabolic reprogramming in cancer, and how these metabolic changes in cancer can be targeted for therapy.
The following are the main research directions of our lab:
- We are interested in investigating novel and critical regulation mechanisms for p53 and its pathway in normal and cancer cells. We have identified new regulators and regulation mechanisms for p53 and oncogenic mutant p53, such as novel E3 ubiquitin ligases, microRNAs, as well as SUMOylation and Palmitoylation modifications that regulate p53. In addition to cell cycle arrest and apoptosis, p53 also regulates many other crucial cellular processes. We are particularly interested in studying how p53 regulates metabolism and anti-tumor immunity, contributing to its role in tumor suppression.
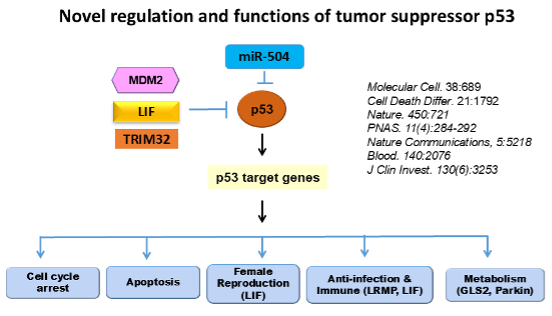
- Tumor-associated mutant p53 proteins, particularly “tumor hotspot mutants”, often gain new activities to promote tumorigenesis. We are interested in studying the mechanisms underlying the oncogenic effects of mutant p53, and how mutant p53 can be targeted for cancer therapy.
- Metabolic reprogramming plays a key role in tumorigenesis and is an attractive target for cancer therapy. We are interested in studying the mechanisms underlying metabolic reprogramming in cancer and how metabolic changes in cancer can be targeted for therapy. We are also interested in studying the non-canonical function of metabolic enzymes in cancer and therapy.