
Hatem E. Sabaawy, MD, PhD
New Brunswick, N.J. – August 12, 2020 – Investigators at Rutgers Cancer Institute of New Jersey have developed a novel approach for utilizing 4-dimensional (4D) printing of arrays that transform from cell-culture inserts into histological cassettes that hold patient tissue samples for rapid programmable drug testing. The arrays were used to process patient derived organoids of a common type of brain tumor, reducing the average testing time by several weeks.
Rutgers Cancer Institute of New Jersey resident research member Hatem E. Sabaawy, MD, PhD, an associate professor of medicine and pathology and laboratory medicine at Rutgers Robert Wood Johnson Medical School is the senior author of the work under a subcontract from Leidos Biomedical Research, Inc., which operates the Frederick National Laboratory for Cancer Research on behalf of the National Cancer Institute. Dr. Sabaawy shares more about the project published online ahead of the August 21 print edition of iScience (doi: 10.1016/j.isci.2020.101365.)
Why is this topic important to explore?
The most common primary brain tumor, glioblastoma multiforme (GBM), is a deadly tumor and even with the best current therapies, patients have a median survival rate of approximately 15 months. GBM does not spare young or old, men or women, or any demographic group. Current treatments including surgery and radiation are not usually effective, as these tumors frequently recur. Even genomic testing (precision medicine) and immunotherapy approaches are not yet beneficial.
With that, there is a need for patient-derived models that may be used to rapidly predict treatment responses and test new and combined therapies that are currently lacking. Due to GBM complexity, it will be critical that such models be used to implement personalized therapy and assess the co-targeting of multiple genomic alterations in GBM for combination therapy.
Tell us about your team’s work and what you discovered.
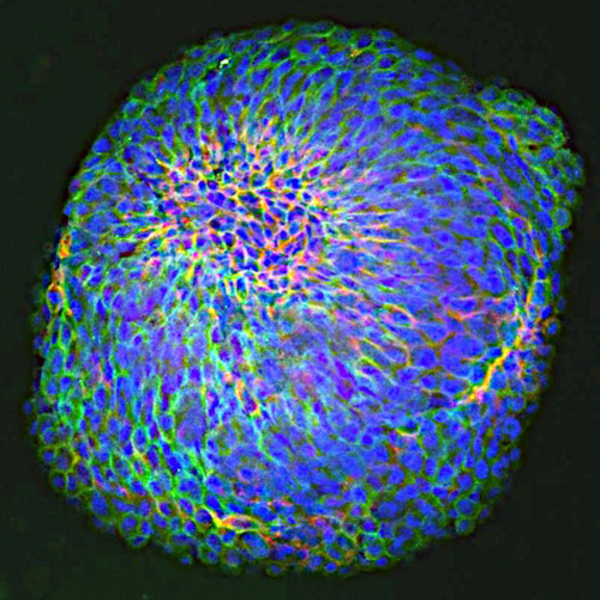
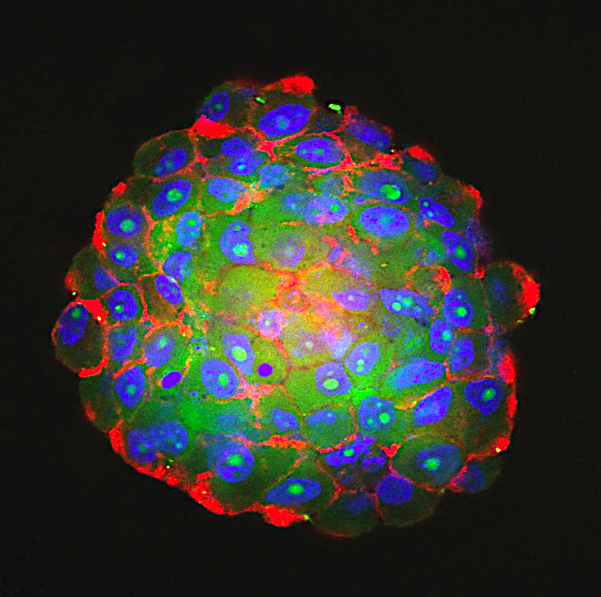
First, the team developed a novel bioengineering approach for utilizing 4-dimensional (4D) printing of arrays that self-transform from cell-culture inserts into histological cassettes for rapid programmable drug testing. Second, the 4D arrays were validated in processing GBM patient derived organoids (PDOs) that were recently developed at Rutgers Cancer Institute. PDOs are cultures of tumor cells derived from patient tissue. These PDOs allowed for determining how effective and synergistic GBM combination therapy may be for each patient’s tumor. The generation of PDOs for drug testing is laborious and lengthy, requiring multiple steps and months to complete.
We developed a 20-day assay for drug testing and assessing synergy between targeted therapies in GBM PDOs. We showed that GBM PDOs contain multiple cell phenotypes, similar to the key tumor cell phenotypes in GBM patients. We identified targeted therapies and combination therapies that could be more effective than the standard of care, first-line therapy temozolomide using PDOs.
What are the implications for future treatments?
We used clonal GBM PDOs to model the genetic subtypes of GBM, by identifying a molecular signature for each subtype, and assessing the effects of treatment with temozolomide and/or combinations of molecularly-targeted agents. Treatment of the GBM PDOs from certain subtypes in the 4D arrays allowed for the identification of possibly effective combination therapies.
The studies utilizing GBM PDOs also revealed that drugs targeting DNA Damage Response and PI3K/mTOR signaling acted synergistically, and might protect from temozolomide toxicity. Intriguingly, we found synergy between drugs at lower than their expected tumor inhibitory concentrations, therefore we think that combination therapy could be used to spare the dose-limiting toxicity in GBM therapy.
What are next steps related to this work?
The findings of synergistic drug combinations could have immediate implications for testing in ongoing and planned clinical trials in GBM and potentially other tumor types. Future research studies will focus on developing different PDOs from other tumor types using 4D printing and other approaches, and including cellular elements of the tumor microenvironment. This integration would enable monitoring of tumor multicellular interactions and assessment of immune and metabolic responses of PDOs to molecularly targeted and immune checkpoint blockade therapy. The team plans to develop further the clinical applications and enhance scaling of the assay to be applied as a CLIA-certified diagnostic test for the evaluation of therapeutic responses to chemotherapeutic, targeted and immunotherapy agents. The team proposes to advance the newly developed assay to be used, once validated, to inform treatment decisions in future precision medicine clinical trials and to select more effective personalized therapies.
For additional information on funding and declaration of interests, visit: doi: 10.1016/j.isci.2020.101365.
###
For journalists – contact:
Krista Didzbalis
Media Relations Assistant
908-812-6114
krista.didzbalis@rutgers.edu
For patient appointments/inquiries – contact:
844-CANCERNJ (844-226-2376)

