At Rutgers Cancer Institute, our experts are among the nation’s most experienced in treating blood cancers with immunotherapy and other cellular therapies, particularly the breakthrough cancer treatment CAR T-Cell therapy.
What is CAR T-Cell therapy and how does it work?
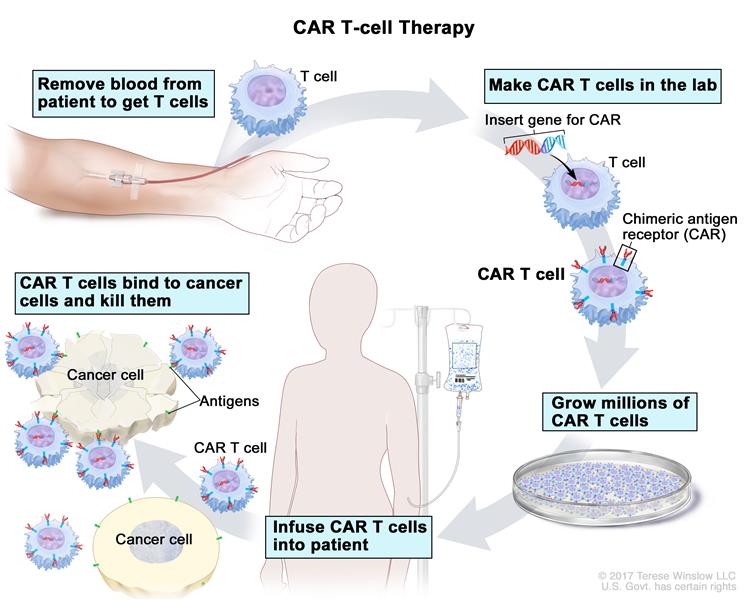
CAR T-Cell therapy is a type of immunotherapy that uses a patient’s own immune cells to fight cancer. Doctors collect T-cells from a patient’s blood through an infusion-like process called apheresis, and the cells are sent to a laboratory. In the lab, scientists modify the T-cells by adding a special receptor called chimeric antigen receptor (CAR) which enables the modified T-cells to seek out and kill cancer cells. The patient’s reengineered CAR T-Cells are returned to them through an infusion, and the cells begin to multiply and attach to cancer cells to destroy them.
What types of cancer are treated with CAR T-Cell therapy?
Currently, CAR T-Cell therapy is approved by the FDA for:
- Adults with relapsed or refractory multiple myeloma
- Adults with relapsed or refractory B-cell lymphomas, including diffuse large B-cell lymphoma, mantle cell lymphoma, follicular lymphoma, and chronic lymphocytic leukemia.
- Adults and children with relapsed or refractory acute lymphoblastic leukemia.
Why choose Rutgers Cancer Institute for CAR T-Cell therapy?
Rutgers Cancer Institute is one of the few institutions in the United States and the only in New Jersey to offer CAR T-Cell therapy at an NCI-designated Comprehensive Cancer Center. Our center is accredited to perform this therapy by the Foundation for the Accreditation of Cellular Therapy (FACT) We are one of only a select few institutions globally that maintains our own Good Manufacturing Practice (GMP) CAR-T manufacturing laboratory, and we design and run next-generation CAR-T clinical trials across a range of illnesses. And our team of clinicians includes world-renown CAR-T experts.
Our clinicians lead the way in knowledge, expertise, and experience in administering highly specialized and advanced therapies to treat benign and malignant blood disorders. Whether you receive an FDA approved therapy or a CAR T-cell clinical trial, we individualize your care to offer you the best possible outcome.
What are some advantages of CAR T-Cell therapy?
One of the major advantages of CAR T-Cell therapy is the short treatment time needed – administered with a single infusion that may require at the most a brief hospital stay, and then it’s done. Because aggressive chemotherapy is not used, most patients have a much more rapid recovery than after stem cell transplants which require more intensive chemotherapy. At some point in the future, CAR T-Cell therapy may even replace most types of transplants but at the current time, CAR T-Cell therapy is approved for the treatment of patients in whom transplant is not likely to be curative or in patients who experience relapse after transplant.
Clinical trials in blood cancers have shown that even in patients whose cancer came back after multiple treatments, CAR T-Cell therapy helped them achieve remissions that lasted for years or even cured their disease. Others can live longer without their cancer getting worse, and in some cases can then benefit from other curative treatments such as stem cell transplantation.
CAR T-Cell therapy is also a “living drug”, and its benefits can last for many years. Since the cells can persist in the body long-term, they may recognize and attack cancer cells if and when there’s a relapse. The data are still evolving, but as many as a third of patients with relapsed aggressive B-cell lymphomas may be cured by CAR T-Cell therapy. And two-thirds of childhood acute lymphoblastic leukemia patients were still in remission after six months. These are patients whose cancers were deemed very aggressive and for whom other standards of care had already failed.
What are the side effects of CAR T-Cell therapy?
The most common side effect of CAR T-Cell therapy is called cytokine release syndrome, or CRS. It’s also known as a “cytokine storm.” Most patients experience it shortly after treatment, but it generally only lasts up to five to seven days. Most patients describe it as having a severe case of the flu, with high fever, fatigue and body aches. It usually starts around the second or third day after the infusion. It happens because the T cells have been multiplying and attacking the cancer, causing an immune response in the body. There are effective medicines to treat CRS, and most patients will have their CRS go away quickly with treatment.
There are other common side effects of CAR T-Cell therapy including brain and nervous system changes (ICANS, or immune effector-cell associated neurological syndrome. Patients can become confused and disoriented and sometimes may not be able to speak at all for a few days. CRES can be upsetting for patients and their families, but it typically is completely reversible. Other known side effects include risks of lowering the immune system, and bone marrow inflammation or suppression. All of these side effects are increasingly well understood and manageable at an NCI-designated Comprehensive Cancer Center such as the Rutgers Cancer Institute.
Read more about receiving CAR T-Cell therapy at Rutgers Cancer Institute and Robert Wood Johnson University Hospital.
Related News
Transforming Cancer Care Through Cellular Therapy and Compassionate Nursing
Nicole McEntee, MSN, RN, OCN, BMTCN, Program Director of the Transplant & Cell Therapy Program at Rutgers Cancer Institute and RWJBarnabas Health, shares how compassionate nursing plays a key role in our cellular and gene therapy program, noting the state-of-the-art, patient-centered care provided at the Jack & Sheryl Morris Cancer Center.
CAR T-Cell Therapy via a Community Lens
Efforts to bring CAR T-cell therapy into the community must address logistical, operational, and educational barriers to improve access for all patients.
Learn more about CAR T-cell therapy: an immunotherapy treatment.

Tennica Johnson Campbell found hope in a CAR T-cell therapy clinical trial


